The FDA plays a critical role in our nation’s response to the COVID-19 pandemic. It helps decide which medicines are safe and effective to treat the disease. It evaluates the accuracy and reliability of laboratory tests. And it will play a central role in assessing potential vaccines.
But will the FDA bureaucracy move with the kind of speed necessary to deal with such a crisis? Some critics are concerned it will not. Even before the rush of the pandemic, the Agency was accused of being slow to evaluate drugs; critics also complained that it denied access to life-altering medications for desperately ill patients.
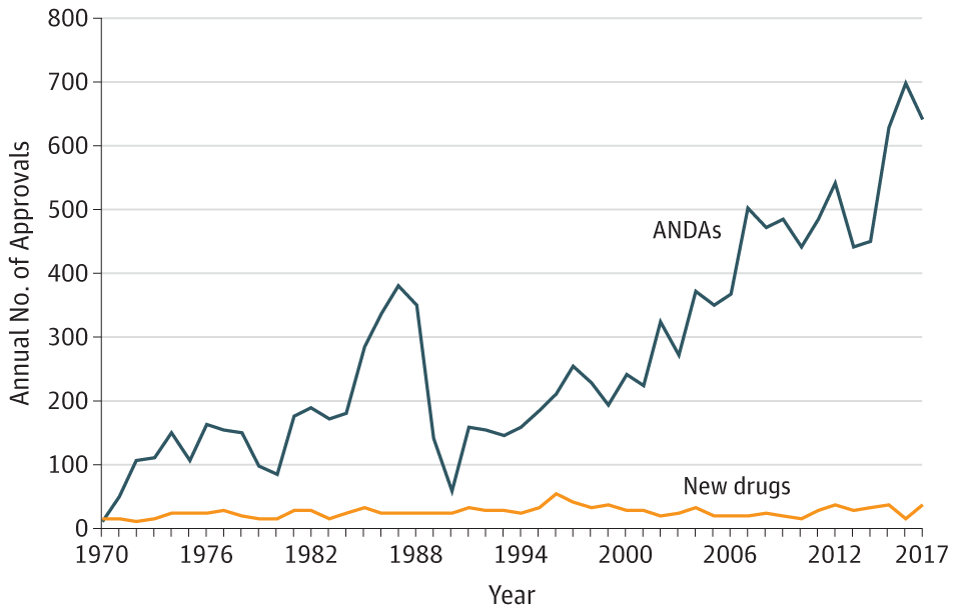
If it were still the 1970s, these criticisms would be well placed. Back then, it took more than three years, on average, for the FDA to review drugs. Review time is now less than one year. Back in the 1970s, it was rare for pharmaceutical companies to obtain expedited review for new medications. Now, these kind of Abbreviated New Drug Applications – or ANDAs – are quite common.
To read the rest of this article, please visit Forbes.